Respond to: Change Management is a formal system for taking care of modifications to processes, specs, or gear Employed in production. QA plays a vital job:
So remember to make sure you provide them with your comprehensive effort and a focus. This features putting absent your e-mail, phone, and Apple Enjoy text messages (not even kidding…) because we could see the secretive eye glances down, and it hurts our souls.
My encounter has also enabled me to establish potent conversation abilities which makes it possible for me to effectively clarify intricate info in a means that is easy to grasp. Furthermore, I am generally keen to learn more about new developments in the field so I can keep up-to-date on the most up-to-date solutions and drugs obtainable.”
A straightforward solution…inquire! And that i’m not declaring you'll want to talk to your preceptor, “Do I have to direct The subject discussions?”
Employers request this question To find out more regarding your qualifications and tips on how to lead for their corporation. Just before your interview, make an index of the abilities and experiences which make you a super applicant for this role. Deal with highlighting your appropriate expertise and tender expertise.
serious. But we’re sort A in pharmacy, and we don’t like making undesirable impressions or not knowing factors, ideal? Hence the shame feels incredibly real.
At last, and this is such as the cherry on leading, the additional credit… Remaining prepared click here signifies understanding if there’s any existing “buzz” with regard to the subject.
Common Pharmaceutical Analyst website interview questions, how to answer them, and case in point answers from a Qualified profession coach.
Your preceptor may well step up to a whiteboard to perform some detailing, or he may expect you to have up for the whiteboard and clarify. It's possible it’ll be a mix of the two.
Investigating deviations and non-conformances: They discover and handle potential excellent issues promptly.
I’ve utilized HPLC for your separation, identification, and quantification of each ingredient in a mix. It’s specifically helpful when dealing with complex Organic samples.
Go through, give your responses, look for clarifications and make use of the discussions for trainings and audit readiness at your facility.
Continual enhancement involves consistently reviewing and updating processes based upon audit conclusions and changes in laws or market ideal tactics. This proactive solution makes sure we maintain higher specifications and adapt properly to any improvements in GMP prerequisites.”
Source allocation: Directing means to controlling important risks instead of spreading them skinny.
 Brian Bonsall Then & Now!
Brian Bonsall Then & Now!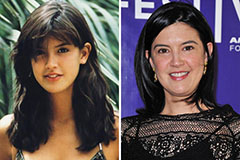 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!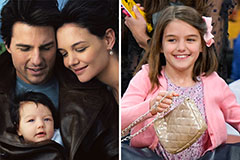 Suri Cruise Then & Now!
Suri Cruise Then & Now! Daryl Hannah Then & Now!
Daryl Hannah Then & Now! Catherine Bach Then & Now!
Catherine Bach Then & Now!